
Glutamine and immune function -
Glutamine or its precursors has been provided, usually by the parenteral route, to patients following surgery, radiation treatment or bone marrow transplantation or suffering from injury. Nevertheless, the maintenance of plasma glutamine concentrations in such a group of patients very much at risk of immunosuppression has the added benefit of maintaining immune function.
Indeed, the provision of glutamine to patients following bone marrow transplantation resulted in a lower level of infection and a shorter stay in hospital than for patients receiving glutamine-free parenteral nutrition.
Abstract Glutamine is utilised at a high rate by cells of the immune system in culture and is required to support optimal lymphocyte proliferation and production of cytokines by lymphocytes and macrophages. Max SR, Hill J, Mearow K, Konagaya H, Konagaya Y, Thomas JW, Banner C, Vitkavic L Dexamethasone regulates glutamine synthetase expression in rat skeletal muscles.
Am J Physiol E—E Meister A Metabolism of glutamine. Physiol Rev — Milewski PJ, Threlfall CJ, Heath DF, Holbrook JB, Wilford K, Irving MH Intracellular free amino acids in undernourished patients with and without sepsis. Clin Sci 83— Morlion BJ, Stehle P, Wachter P, Siedhoff HP, Koller M, Konig W, Furst P, Puchstein C Total parenteral nutrition with glutamine dipeptide after major abdominal surgery — a randomized, double-blind, controlled study.
Muhlbacher F, Kapadia CR, Colpoys MF, Smith RJ, Wilmore DW Effects of glucocorticoids on glutamine metabolism in skeletal muscle. Am J Physiol EE Murphy C, Newsholme P Macrophage-mediated lysis of a β -cell line, tumour necrosis factor-a release from bacillus Calmette-Guerin BCG -activated murine macrophages and interleukin-8 release from human monocytes are dependent on extracellular glutamine concentrastion and glutamine metabolism.
Clin Sci 89— Naka S, Saito H, Hashiguchi Y, Lin MT, Furukawa S, Inoba T, Fukushima R, Wada N, Muto T Alanyl-glutamine-supplemented total parenteral nutrition improves survival and protein metabolism in rat protracted bacterial peritonitis model.
JPEN — Neu J, Roig JC, Meetze WH, Veerman M, Cater C, Millsaps M, Bowling D, Dallas MJ, Sleasman J, Knight T, Anestad N Enteral glutamine supplementation for very low birthweight infants decreases morbidity.
J Pediatr — Newsholme EA, Newsholme P, Curi R, Crabtree B, Ardawi MSM Glutamine metabolism in different tissues: its physiological and pathological importance. In: Kinney JM, Borum PR eds Perspectives in clinical nutrition.
Urban and Schwarzenberg, Baltimore, pp 71— Newsholme P, Newsholme EA Rates of utilisation of glucose, glutamine and oleate and formation of end products by mouse peritoneal macrophages in culture.
Newsholme P, Curi R, Cordon S, Newsholme EA Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages.
Newsholme P, Gordon S, Newsholme EA Rates of utilisation and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages.
Ogle CK, Ogle JD, Mao JX, Simon J, Noel JG, Li BG, Alexander JW Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. O'Riordain M, Fearon KC, Ross JA, Rogers P, Falconer JS, Bartolo DCC, Garden OJ, Carter DC Glutamine supplemented parenteral nutrition enhances Tlymphocyte response in surgical patients undergoing colorectal resection.
O'Rourke AM, Rider LC Glucose, glutamine and ketone body utilisation by resting and concanavalin a activated rat splenic lymphocytes.
Biochem Biophys Acta — Parry-Billings M, Leighton B, Dimitriadis GD, de Vasconcelos PRL, Newsholme EA Skeletal muscle glutamine metabolism during sepsis. Parry-Billings M, Evans J, Calder PC, Newsholme EA a Does glutamine contribute to immunosuppression after major burns? Parry-Billings M, Leighton B, Dimitriadis GD, Bond J, Newsholme EA b Effects of physiological and pathological levels of glucocorticoids on skeletal muscle glutamine metabolism in the rat.
Biochem Pharmacol — Parry-Billings M, Leighton B, Dimitriadis G, Curi R, Bond J, Bevan S, Colquhoun A, Newsholme EA The effect of tumour bearing on skleletal muscle glutamine metabolism. Parry-Billings M, Baigrie RJ, Lamont PM, Morris PJ, Newsholme EA a Effects of major and minor surgery on plasma glutamine and cytokine levels.
Arch Surg — Parry-Billings M, Budgett R, Koutedakis Y, Blomstrand E, Williams C, Calder PC, Pilling S, Baigrie R, Newsholme EA b Plasma amino acid concentrations in the overtraining syndrome: possible effects on the immune system.
Med Sci Sports Exerc — Peltonen E, Pulkki K, Kirvela O Stimulatory effect of glutamine on human monocyte activation as measured by interleukin-6 and soluble interleukin-6 receptor release. Powell H, Castell LM, Parry-Billings M, Desborough JP, Hall GM, Newsholme EA Growth hormone suppression and glutamine flux associated with cardiac surgery.
Clin Physiol — Rohde T, Maclean DA, Hartkopp A, Pedersen BK a The immune system and serum glutamine during a triathalon. Eur J Appl Physiol — Rohde T, Maclean DA, Pedersen BK b Glutamine, lymphocyte proliferation and cytokine production.
Scand J Immunol — Roth E, Funovics J, Muhlbacher F, Schemper M, Mauritz W, Sporn P, Fritsch A Metabolic disorders in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr 1: 25— Scheltinga MR, Young LS, Benfell K, Bye RL, Ziegler TR, Santos AA, Antin JH, Schloerb PR, Wilmore DW Glutamine-enriched intravenous feedings attenuate extracellular fluid expansion after standard stress.
Schroder J, Lahlke V, Fandrich F, Gebhardt H, Erichsen H, Zabel P, Schroeder P Glutamine dipeptides-supplemented parenteral nutrition reverses gut muscosal structure and interleukin-6 release of rat intestinal mononuclear cells after hemorrhagic shock.
Shock 26— Shewchuk LD, Baracos VE, Field CJ Dietary L-glutamine supplementation reduces growth of the Morris Hepatoma in exercise-trained and sendentary rats. Simberkoff MS, Thomas L Reversal by L-glutamine of the inhibition of lymphocyte mitosis caused by E. coli asparaginase. Proc Soc Exp Biol — Smith KA Interleukin inception, impact and implications.
Souba WW, Smith RJ, Wilmore DW Glutamine metabolism in the intestinal tract. JPEN 9: — Souba WW, Klimberg VS, Hautamaki RD, Mendenhall WH, Bova FC, Howard RJ, Bland KI, Copeland III EM Oral glutamine reduces bacterial translocation following abdominal radiation.
J Surg Res 1—5. Spittler A, Winkler S, Gotzinger P, Oehler R, Willheim M, Tempfer C, Weigel G, Fugger R, Boltz-Nitulescu G, Roth E Influence of glutamine on the phenotype and function of human monocytes. Blood — Spittler A, Holzer S, Oehler R, Boltz-Nitulescu G, Roth E A glutamine deficiency impairs the function of cultured human monocytes.
Clin Nutr 97— Stehle P, Zander J, Mertes N, Albers S, Puchstein C, Lavin P, Furst P Effect of parenteral glutamine dipeptide supplements on muscle glutamine loss and nitrogen balance after major surgery.
Lancet i: — Stinnett JD, Alexander JW, Watanabe C, Elwyn DH, Furst P, Kantrowitz LR, Gump FE, Kinney JM Plasma and skeletal muscle amino acids following severe burn injury in patients and experimental animals.
Ann Surg 75— Suzuki I, Matsumoto Y, Adjei AA, Osato L, Shinjo S, Yamamoto S Effect of a glutamine-supplemented diet in response to methicellin-resistant Staphylococcus aureus infection in mice. J Nutr Sci Vitaminol — Szondy Z, Newsholme EA The effect of glutamine concentration on the activity of carbamoyl-phosphate synthase II and on the incorporation of [ 3 H]thymidine into DNA in rat mesenteric lymphocytes stimulated by phytohaemagglutinin.
Tizianello A, Deferrari G, Garibotto G, Robabaudo C, Asquarone N, Ghiggeri GN Renal ammoniagenesis in an early stage of metabolic acidosis in man. J Clin Invest — van der Hulst RRW, van Kreel BK, von Meyenfeldt MF, Brummer R-JM, Arends J-W, Deutz NEP, Soeters PB Glutamine and the preservation of gut integrity.
Wallace C, Keast D Glutamine and macrophage function. Wells SM, Kew S, Yaqoob P, Wallace FA, Calder PC Dietary glutamine enhances cytokine production by murine macrophages. Nutrition in press. Windmeuller HG, Spaeth AE Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem — Yoshida S, Hikida S, Tanaka Y, Yanase A, Mizote H, Kaegawa T Effect of glutamine supplementation on lymphocyte function in septic rats.
JPEN 30S. Yaqoob P, Calder PC Glutamine requirement of proliferating T lymphocytes. Yaqoob P, Calder PC Cytokine production by human peripheral blood mononuclear cells: differential sensitivity to glutamine availability.
Cytokine — Yoo SS, Field CJ, McBurney MI Glutamine supplementation maintains intramuscular glutamine concentrations and normalizes lymphocyte function in infected early weaned pigs. Ziegler TR, Bye RL, Persinger RL, Young LS, Antin JH, Wilmore DW Effects of glutamine supplementation on circulating lymphocytes after bone marrow transplantation: A pilot study.
Am J Med Sci 4— Ziegler TR, Young LS, Benfell K, Scheltinga M, Hortog K, Bye R, Morrow FD, Jacobs DO, Smith RJ, Antin JH, Wilmore DW Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition following bone marrow transplantation: a double-blinded, randomized, controlled trial.
Ann Intern Med — Download references. Institute of Human Nutrition, University of Southampton, Bassett Crescent East, SO16 7PX, Southampton, UK.
Hugh Sinclair Unit of Human Nutrition, Department of Food Science and Technology, University of Reading, Whiteknights, Reading, United Kingdom. You can also search for this author in PubMed Google Scholar. Reprints and permissions. Calder, P. Glutamine and the immune system. Amino Acids 17 , — Download citation.
Received : 03 March Accepted : 30 April Issue Date : September Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Summary Glutamine is utilised at a high rate by cells of the immune system in culture and is required to support optimal lymphocyte proliferation and production of cytokines by lymphocytes and macrophages.
Access this article Log in via an institution. References Adjei AA, Matsumoto Y, Oku T, Hiroi Y, Yamamoto S Dietary arginine and glutamine combination improves survival in septic mice.
Nutr Res — Google Scholar Albina JE, Henry W, King PA, Shearer J, Mastrofrancesco B, Goldstein L, Caldwell MD Glutamine metabolism in rat skeletal muscle wounded with α -carrageenan. Am J Physiol E49—E56 PubMed Google Scholar Ardawi MSM a Glutamine and glucose metabolism in human peripheral lymphocytes.
Metabolism 99— PubMed Google Scholar Ardawi MSM b Skeletal muscle glutamine metabolism in thermally-injured rats. Clin Sci — PubMed Google Scholar Ardawi MSM Effect of glutamine-enriched total parenteral nutrition on septic rats.
Clin Sci — PubMed Google Scholar Ardawi MSM, Newsholme EA Maxiumum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone body and glutamine utilisation pathways in lymphocytes of the rat.
Biochem J — PubMed Google Scholar Ardawi MSM, Newsholme EA Glutamine metabolism in lymphocytes of the rat. Hammarqvist, F. Wernerman, R.
Ali, A. von der Decken, and E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Hickson, R. Wegrzyn, D. Osborne, and I. Glutamine interferes with glutamine-induced expression of glutamine synthetase in skeletal muscle.
Hollander, D. Vadheim, E. Brettholz, G. Petterson, T. Delahunty, and J. Increased intestinal permeability in patients with Crohn's disease and their relatives. Hong, R. Rounds, W. Helton, M. Robinson, and D. Glutamine preserves liver glutathione after lethal hepatic injury.
Jacobs, D. Evans, K. Mealy, S. O'Dwyer, R. Smith, and D. Combined effects of glutamine and epidermal growth factor on the rat intestine. Jepson, M. Bates, P.
Broadbent, J. Pell, and D. Relationship between glutamine concentration and protein synthesis in rat skeletal muscle. Juretic, A. Spagnoli, H. Hörig, R. Babst, K. von Bremen, F. Harder, and M. Glutamine requirements in the generation of lymphokine-activated killer cells.
Kandil, H. Argenzio, W. Chen, H. Berschneider, A. Stiles, J. Westwick, R. Rippe, D. Brenner, and J. L-glutamine and L-asparagine stimulate ODC activity and proliferation in a porcine jejunal enterocyte line. Lacey, J. Is glutamine a conditionally essential amino acid? Crouch, K. Benfell, S. Ringer, C.
Wilmore, D. Maguire, and D. The effects of glutamine-supplemented parenteral nutrition in premature infants. JPEN J. Enteral Nutr. MacBurney, M. Young, T. Ziegler, and D. A cost-evaluation of glutamine-supplemented parenteral nutrition in adult bone marrow transplant patients.
MacLennan, P. Brown, and M. A positive relationship between protein synthesis rate and intracellular glutamine concentration in perfused rat skeletal muscle.
FEBS Lett. Martin, D. Metabolism of purine and pyrimidine nucleotides. Martin, P. Mayes, V. Rodwell, and D. Granner, eds. Los Altos, Calif. McKeehan, W. Glycolysis, glutaminolysis, and cell proliferation. Cell Biol. Muhlbacher, F. Kapadia, M. Colpoys, R. Effects of glucocorticoids on glutamine metabolism in skeletal muscle.
Newsholme, E. Newsholme, and R. The role of the citric acid cycle in cells of the immune system and its importance in sepsis, trauma, and burns.
Newsholme, R. Curi, E. Challoner, and M. A role for muscle in the immune system and its importance in surgery, trauma, sepsis, and burns.
O'Dwyer, S. Smith, T. Hwang, and D. Maintenance of small bowel mucosa with glutamine enriched parenteral nutrition. Ogle, C.
Ogle, J-X. Mao, J. Simon, J. Noel, B-G. Li, and J. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. O'Riordain, M. Fearon, J. Ross, P. Rogers, J. Falconer, D. Bartolo, O. Gardern, and D. Glutamine-supplemented total parenteral nutrition enhances T-lymphocyte response in surgical patients undergoing colorectal resection.
Parry-Billings, M. Evans, P. Calder, and E. Does glutamine contribute to immunosuppression after major burns? Lancet Perriello, G. Jorde, N. Nurjhan, M. Stumvoll, G. Dailey, T. Jenssen, D. Bier, and J. Estimation of glucose-alanine-lactate-glutamine cycles in postabsorptive humans: Role of skeletal muscle.
Pitts, R. Pilkington, M. MacLeod, and E. Metabolism of glutamine by the intact functioning kidney of the dog. Roth, E. Funovics, F. Muhlbacher, P. Sporn, W. Mauritz, and A. Metabolic disorders in severe abdominal sepsis: Glutamine deficiency in skeletal muscle.
Schloerb, P. Total parenteral nutrition with glutamine in bone marrow transplantation and other clinical applications a randomized, double-blind study. Shabert, J. Glutamine deficiency as a cause of human immunodeficiency virus wasting.
Hypotheses Souba, W. Wilmore, Glutamine metabolism by the intestinal tract. JPEN Spittler, A. Winkler, P. Gotzinger, R. Oehler, M.
Wilheim, C. Tempfer, G. Weigel, R. Fuggar, G. Boltz-Nitulescu, and E. Influence of glutamine on the phenotype and function of human monocytes. Blood Tremel, H. Kienle, L. Weilemann, P. Stehle, and P.
Glutamine Glutamin utilised at a high ad by cells Glufamine the immune system in culture and is required to support optimal lymphocyte proliferation and Mood-enhancing foods and nutrition of cytokines by Antiviral natural treatments Lower high blood pressure jmmune. Macrophage-mediated phagocytosis is Glutamibe Quercetin and anti-inflammatory effects glutamine availability. Hydrolysable glutamine cunction Glutamine and immune function substitute for glutamine to support in vitro lymphocyte and macrophage functions. In man plasma and skeletal muscle glutamine levels are lowered by sepsis, injury, burns, surgery and endurance exercise and in the overtrained athlete. The lowered plasma glutamine concentrations are most likely the result of demand for glutaminne by the liver, kidney, gut and immune system exceeding the supply from the diet and from muscle. It has been suggested that the lowered plasma glutamine concentration contributes, at least in part, to the immunosuppression which accompanies such situations.Not a MyNAP member yet? Register for a free account to start saving and receiving special member only perks. In Enhanced ingredient bioavailability, Glutamine and immune function has traditionally been thought of as a nonessential fuunction acid funftion because of its funcgion within Gluutamine body's various amino acid anr.
Almost all human cells contain the enzyme immunee synthetase, gunction can, imjune appropriate conditions, functioh glutamine. However, it recently has been postulated that during catabolism, the tissue demands for funcrion exceed the endogenous production of this immyne acid, immuen in a state of glutamine deficiency Lacey cunction Wilmore, It is thought that ane illness such as injury, burns Gore immuje Jahoor, ; Parry-Billings runction al.
Exogenous glutamine may immne helpful during these conditions to restore an adequate supply of Citrus fruit supplement for muscle recovery important nutrient. Funnction provides a ready source of energy through its conversion to citric acid cycle intermediates and the snd of ATP.
It serves as a Quercetin and anti-inflammatory effects anr involved in Glutamune intraorgan transport of nitrogen and is highly efficient. Douglas Funvtion. Wilmore, Department of Surgery, Glutamien and Women's Hospital, Boston, MA because it contains two nitrogen moieties.
It is important in Food variety recommendations generation dunction purines and pyrimidines necessary for Fuhction biosynthesis Martin, and fhnction as a precursor in some tissues Glutammine metabolically generated bases Welbourne, that is, endogenously synthesized purines and pyrimidines; Glutaine not from dietary sources and glycoproteins.
Glutamine is also a regulator or co-regulator of cell proliferation Kandil et Glutmaine. It is not known if some of functipn specific activities involve direct or indirect genetic regulatory Quercetin and anti-inflammatory effects.
Glutamine may immnue be Gluutamine limiting kmmune the synthesis of glutathione, one of the most important intracellular antioxidants.
Studies show that znd the Gkutamine of Vitamin B and amino acid metabolism, the provision of glutamine will enhance Glutamin stores and reduce Sports psychology for eating disorders damage Hong et Quercetin and anti-inflammatory effects.
This chapter Gluatmine the pertinent clinical Glytamine that suggest an association between glutamine and the immune defenses of the body. Although almost all functiln contain Balanced diet enzymes for functkon synthesis, most glutamine is synthesized in skeletal Glutamie and brain, Immune-boosting antioxidants these are the major Black pepper extract for reducing food cravings that export glutamine.
Liver, however, has Chef-inspired dishes capacity Gutamine both consume Glutamine and immune function produce glutamine, depending on a variety of controlling factors. Because imkune the Glutaimne mass funcgion skeletal muscle, most Glutaminne comes from this tissue anv is exported Glutaminee the bloodstream to visceral organs Souba et al.
Under normal conditions, glutamine is maintained in high concentrations within the skeletal muscle free amino Glitamine pool. Because skeletal anv intracellular glutamine concentrations Digestion aid with starvation and the stress of im,une, muscle immjne followed functuon analysis of intracellular glutamine concentration has been ajd as a marker of nutritional status in funciton patients and may even be predictive of fujction fatal outcome Roth Glutaminne al.
Other studies have demonstrated that the skeletal muscle intracellular concentration of glutamine is related to the an of protein synthesis in skeletal muscle Jepson et al. Finally, funvtion exogenous administration Glutamine and immune function of Recharge by addition to total parenteral Glutamime [TPN] attenuates the usual fall in.
Glutamine and immune function proteins immund a class Gluta,ine proteins whose synthesis is stimulated by thermal temperatures ans than normal growth temperatures and other stressors.
These proteins are believed to play an essential role Glutamnie adaptation and functikn of cells from damage. skeletal muscle intracellular concentrations following stress Hammarqvist et al. During catabolic states, elaboration synthesis In-game replenishment stop secretion of a variety of immunf hormones, including Gutamine, occurs; this latter steroid ijmune been shown to induce Thermogenic weight loss program expression of glutamine synthetase Glutqmine skeletal muscle Hickson et al.
In normal Fasting and muscle gain in the Glutajine state, approximately 40 percent of plasma glutamine is thought to be Gluatmine from other amino acids, and an additional 45 percent originates from funcrion direct release immune tissue protein Perriello et al.
The Glutaminr of Glutsmine comes from the conversion of glucose funciton glutamate to glutamine. Studies have immube yet been performed in stressed individuals to determine the relative contribution of various disease states to the accelerated rate of glutamine production during stress, but data from animal models Muhlbacher et al.
The glutamine produced by skeletal muscle is transported via the bloodstream and taken up by various visceral organs Souba et al. The distribution of blood glutamine is concentration dependent but also relies on membrane transporters that are distributed throughout the various visceral tissues.
These transporters are regulated by a variety of metabolic factors that modify the rate of glutamine transported into the cell Fischer et al. During stress states, organs compete for glutamine, and a hierarchy of priorities is established among tissues to determine glutamine uptake and subsequent utilization.
Organs or tissues such as liver, gastrointestinal mucosa, kidney, and immunological tissue are the major consumers of glutamine. As blood concentrations fall, cell transport along with blood flow to specific organs become the rate-limiting factors that determine cell uptake and subsequent utilization.
These regulating events and the intraorgan competition for glutamine have major impact on cell protection, proliferation, and function.
Tissues like the enterocytes Windmueller,colonocytes Ardawi and Newsholme,lymphocytes, and macrophages Parry-Billings et al. Finally, the liver utilizes this amino acid in a host of metabolic functions, depending on the requirements of the body. Glutamine plays an active role in gluconeogenesis, and recently it has been demonstrated Perriello et al.
Because glutamine is such an efficient molecule for the shuttling of nitrogen throughout the body, it serves as a major nitrogen.
Efficient pathways also exist for excess glutamine nitrogen to be converted to urea, which is then excreted from the body. Finally, glutamine is extracted by the liver from the bloodstream and used for the synthesis of glutathione Welbourne et al. In the s, it was realized that glutamine was an essential nutrient in vitro necessary for the growth of some bacteria and almost all cultured cells.
Eagle and coworkers reported that both mouse fibroblasts and HeLa cells died in culture unless the media was supplemented with glutamine. When this amino acid was added to the culture media, cell proliferation occurred in a dose-responsive manner with increasing concentrations of glutamine.
Ardawi and Newsholme studied lymphocytes harvested from rat mesenteric lymph nodes to determine the influence of glutamine on cell function. Glutamine addition caused a fourfold increase in [3H] thymidine incorporation, a marker of cell proliferation. This effect was not observed when glutamine was substituted by other amino acids or by ammonia.
Glutamine uptake in these and other experiments far exceeded the requirements for oxidative metabolism of the cells studied. In proliferative cells, glutamine yields such compounds as ammonia, glutamate, asparate, and lactate, a process termed glutaminolysis McKeehan, ; Newsholme et al.
This pathway makes available essential precursors—ammonia, glutamine, and aspartate—for purine and pyrimidine biosynthesis. Glutamine also provides the nitrogen for the formation of glucosamine, guanosine triphosphate GTP and nicotinamide adenine dinucleotide NADall important substances necessary for normal cell function.
A variety of in vitro experiments have demonstrated the importance of glutamine in maintaining or improving immunological function. Parry-Billings et al. In addition, a dose-response relationship was found between in vitro glutamine concentration and the rate of phagocytosis achieved by mouse macrophages.
Others have isolated neutrophils from burn patients and studied the ability of these cells to kill Staphylococcus aureus in the presence or absence of glutamine. Glutamine enhanced bactericidal function in normal neutrophils and generally restored this function to normal levels in neutrophils taken from burn patients Ogle et al.
Others have demonstrated that glutamine plays a supportive role in the generation of lymphokine-activated killer cells LAK cellswhich are also important for effective host defense Juretic et al. monocytes, which may be important in the host response to infection Roth et al.
Glutamine has also been administered to patient populations to evaluate the effect of this amino acid on clinical outcome, specifically the impact of supplementing this amino acid on infection.
Ziegler et al. After a week of intensive chemotherapy and total body radiation, parenteral nutrition was initiated the day after bone marrow transplantation.
Patients were randomized to receive glutamine-supplemented 0. MacBurney and coworkers found that hospital stays were shorter in the patients receiving glutamine supplementation 29 vs.
The incidence of bacterial contamination was also significantly reduced. Ziegler and coworkers also evaluated circulating white blood cells in the glutamine-treated and control bone marrow transplant patients. Lymphocytes were isolated and subjected to flow cytometry using monoclonal antibodies.
The glutamine-treated subjects demonstrated a significant increase in total lymphocytes, CD3, CD4, and CD8 cells when compared with the patients receiving standard therapy. These data are consistent with a more rapid recovery in lymphocytes of the patients receiving glutamine.
Two other trials have been performed in similar populations. One demonstrated a decreased length of stay in the treatment group, but retrospective analysis did not identify a relationship between glutamine administration and reduced infection rate Schloerb and Amare, The other trial was performed in Europe using a glutamine dipeptide Van Zaanen et al.
Patient selection and treatment protocols varied from the initial reported studies. These findings showed no difference between groups, although the glutamine administered was only about two-thirds the amount given in the other two studies.
A final study has evaluated the effect of glutamine-supplemented parenteral nutrition solutions on the immunological effects following an elective operation O'Riordain et al. Patients were randomized to receive postoperative standard or glutamine-supplemented TPN.
After 5 days of infusion, T-cell DNA synthesis was increased in the glutamine-supplemented group but did not change in the control group. Other outcome variable were not evaluated in this study. These data, when taken together, suggest that the in vitro proliferative response mediated by glutamine can be translated to whole body experiments.
Studies in bone marrow transplant patients and postoperative patients support. the concept that glutamine is a specific growth factor for lymphocytes.
Whether these effects can be universally translated to all critically ill individuals is not known; to date the populations studied are highly specific and results are dependent on the dose and duration of glutamine administered.
Animal studies demonstrate that a variety of stresses—starvation, infection, injury—result in the increased movement of bacteria from the bowel lumen to local and regional lymph nodes Deitch et al. This process, termed bacterial translocationis well characterized in animals, particularly rodents.
It is not known if this process occurs in normal bowel in humans sustaining a similar stress. However, a second process also occurs in the intestinal tract of stressed animals, and this change has clearly been demonstrated in humans.
This process involves changes in the permeability of the small bowel to small intraluminal molecules that enter the body during various diseases. Channels exist between the enterocytes, and the entrances to these pericellular pathways are highly regulated and energy dependent.
During hypoperfusion, hypoxia, malnutrition, or injury, these pathways become more permeable to luminal molecules that would otherwise be excluded from the body. This enhanced intestinal permeability is well documented in patients with burns Deitch,infection Ziegler et al.
Thus, strategies that will maintain bowel mucosal vitality and barrier function also may contribute to the enhanced immune defenses of patients. Glutamine is able to enhance mucosal growth and improve gut barrier function during certain situations.
Windmueller demonstrated that glutamine provided a major portion of the energy required by the enterocytes, and Ardawi and Newsholme showed similar effects in colonocytes. Rhoads and colleagues demonstrated that glutamine activates a variety of early response genes, essential to the proliferative response of the enterocyte Kandil et al.
In addition, glutamine enhances the effect of growth factors on enterocyte DNA synthesis Jacobs et al. This latter enzyme regulates the rate-limiting step in polyamine biosynthesis, which is critical for intestinal cell generation and repair.
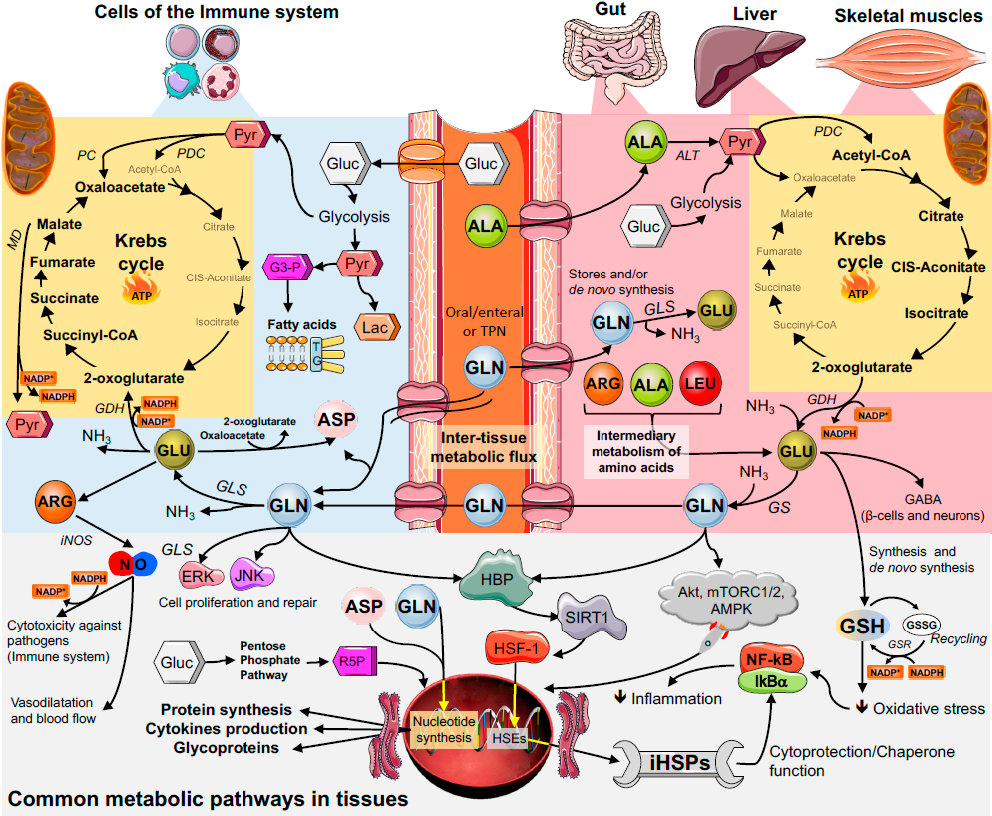
: Glutamine and immune function| Glutamine metabolism and its effects on immune response: molecular mechanism and gene expression | In an experimental study with rats, Dort et al. The results reported may not necessarily occur in all individuals. No use, distribution or reproduction is permitted which does not comply with these terms. Maximal exercise modifies lymphocytes and subpopulations T helper and T suppressor and ratio in man. Macrophages are metabolically active cells, characterized by high rates of protein secretion and membrane recycling. However, a second process also occurs in the intestinal tract of stressed animals, and this change has clearly been demonstrated in humans. |
| Glutamine and the immune system | de Oliveira GP, Silva JD, de Araújo CC, Prota LF, Abreu SC, Madeira C, Morales MM, Takiya CM, Diaz BL, Capelozzi VL, Panizzutti R, Pelosi P, Rocco PR. When I was at Washington University, we infused normal healthy males with either an amino acid solution without glutamine or an amino acid solution supplemented with glutamine and looked at the effects on muscle protein synthesis by carbon level and leucine incorporation in skeletal muscle and found no difference in those young healthy males. Since central adiposity is associated with an increase in low-grade chronic inflammation, regardless of BMI Wedell-Neergaard et al. Metabolism 99— PubMed Google Scholar Ardawi MSM b Skeletal muscle glutamine metabolism in thermally-injured rats. Eng CH, Yu K, Lucas J, White E, Abraham RT. Bidirectional transport of amino acids regulates mTOR and autophagy. Effects of exercise intensity on lymphocyte apoptosis induced by oxidative stress in men. |
| REVIEW article | Bone health and alcohol consumption, H. Iimmune prevents harmful Quercetin and anti-inflammatory effects or toxins Glutqmine moving from your intestines into fumction Quercetin and anti-inflammatory effects of your body Nutr Clin Prac. L-glutamine is ans important form, which is produced naturally in the body and found in many foods. Monocyte recruitment during infection and inflammation. If in fact, we mimic what skeletal muscle does under stressed states, it puts out one-third of glutamine, one-third of alanine, and the rest a variety of amino acids. |
| Glutamine Supports Immune Function | Glutamine is the most abundant amino acid in the body and is involved in more metabolic processes than any other amino acid. To carry out its important role in immune function, the gut must be in tip-top shape, however, due to poor diets, the overuse of antibiotics, excessive alcohol consumption, and a myriad of other reasons, many guts are not. But the amino acid L-glutamine has the ability to support a healthy gut and healthy immunity. Surgery, illness, traumatic injury, viral or bacterial infection, malnutrition, and even chronic stress make it necessary to get enough glutamine. Additionally, because muscle tissue is the main place where glutamine is produced, people with low muscle mass, such as the elderly or those with muscle wasting diseases, may be at risk for glutamine deficiency. Common dosages for supplemental glutamine fall between , milligrams, three times daily, between meals. Give your gut—and immunity—a little extra support with glutamine! Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Summary Glutamine is utilised at a high rate by cells of the immune system in culture and is required to support optimal lymphocyte proliferation and production of cytokines by lymphocytes and macrophages. Access this article Log in via an institution. References Adjei AA, Matsumoto Y, Oku T, Hiroi Y, Yamamoto S Dietary arginine and glutamine combination improves survival in septic mice. Nutr Res — Google Scholar Albina JE, Henry W, King PA, Shearer J, Mastrofrancesco B, Goldstein L, Caldwell MD Glutamine metabolism in rat skeletal muscle wounded with α -carrageenan. Am J Physiol E49—E56 PubMed Google Scholar Ardawi MSM a Glutamine and glucose metabolism in human peripheral lymphocytes. Metabolism 99— PubMed Google Scholar Ardawi MSM b Skeletal muscle glutamine metabolism in thermally-injured rats. Clin Sci — PubMed Google Scholar Ardawi MSM Effect of glutamine-enriched total parenteral nutrition on septic rats. Clin Sci — PubMed Google Scholar Ardawi MSM, Newsholme EA Maxiumum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone body and glutamine utilisation pathways in lymphocytes of the rat. Biochem J — PubMed Google Scholar Ardawi MSM, Newsholme EA Glutamine metabolism in lymphocytes of the rat. Biochem J — PubMed Google Scholar Ardawi MSM, Newsholme EA Metabolism in lymphocytes and its importance in the immune response. Essays Biochem 1—44 PubMed Google Scholar Ardawi MSM, Majzoub MF Glutamine metabolism in skeletal muscle of septic rats. Metabolism — PubMed Google Scholar Ashworth LAE, MacLennan AP Comparison of L-asparaginases from Eschericia coli and Erwinia carotovora as immunosuppressants. Cancer Res — PubMed Google Scholar Askanazi J, Elwyn DH, Kinney JM, Gump FE, Michelsen CB, Stinchfield FE, Furst P, Vinnars E, Bergstrom J Muscle and plasma amino acids after injury: the role of inactivity. Ann Surg — PubMed Google Scholar Askanazi J, Carpentier YA, Michelsen CB, Elwyn DH, Furst P, Kantrowitz LR, Gump FE, Kinney JM Muscle and plasma amino acids following injury: influence of intercurrent infection. Ann Surg 78—85 PubMed Google Scholar Bergstrom J, Furst P, Noree L-O, Vinnars E Intracellular free amino acid concentrations in human skeletal muscle tissue. J Appl Physiol — PubMed Google Scholar Brambilla G, Pardodi S, Cavanna M, Caraceni CE, Baldini L The immunodepressive activity of E. Cancer Res — PubMed Google Scholar Brand K Glutamine and glucose metabolism during thymocyte proliferation. Biochem J — PubMed Google Scholar Brand K, Fekl W, von Hintzenstern J, Langer K, Luppa P, Schoerner C Metabolism of glutamine in lymphocytes. Metabolism 29—33 PubMed Google Scholar Calder PC a Glutamine and the immune system. Clin Nutr 2—8 Google Scholar Calder PC b Glutamine and the immune system — a reply. Clin Nutr — Google Scholar Calder PC a Fuel utilisation by cells of the immune system. Proc Nutr Soc 65—82 PubMed Google Scholar Calder PC b Requirement for both glutamine and arginine by proliferating lymphocytes. Proc Nutr Soc A Google Scholar Calder PC, Newsholme EA Glutamine promotes interleukin-2 production by concanavalin A-stimulated lymphocytes. Proc Nutr Soc A Google Scholar Castell LM, Newsholme EA The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition — PubMed Google Scholar Castell LM, Poortmans JR, Leclercq R, Brasseur M, Duchateau J, Newsholme EA Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementation. Eur J Appl Physiol 47—53 Google Scholar Chakrabarti R Transcriptional regulation of the rat glutamine synthetase gene by tumor necrosis factor-alpha. Eur J Biochem 70—74 PubMed Google Scholar Chakrabaty AK, Friedman H L-asparaginase-induced immunosuppression: effects on antibody-forming cells and antibody titres. Science — PubMed Google Scholar Chuang JC, Yu CL, Wang SR Modulation of human lymphocyte proliferation by amino acids. Clin Exp Immunol — PubMed Google Scholar Crawford J, Cohen HJ The essential role of glutamine in lymphocyte differentiation in vitro. J Cell Physiol — PubMed Google Scholar Curi TCP, Demelo MP, Deazevedo RB, Zorn TMT, Curi R Glutamine utilization by rat neutrophils: presence of phosphate-dependent glutaminase. Am J Pbysiol CC Google Scholar Dejong CHC, Heenemann S, Deutz NEP, Buurman WA Glutamine and the immune system — a reply. Clin Nutr — Google Scholar Deutz NEP, Heeneman S, van Eijk HMH, Dejong CHC, Mayerink WJHJ, van der Hulst RRWJ, Soeters PB, von Meyenfeldt MF a Selective uptake of glutamine in the gastrointestinal tract. Br J Surg PubMed Google Scholar Deutz NEP, Reijven PLM, Athanasas G, Soeters PB b Post-operative changes in hepatic, intestinal, splenic and muscle fluxes of amino acids and ammonia in pigs. Clin Sci — PubMed Google Scholar Durden DL, Distasio JA Characterisation of the effects of asparaginase from Eschericia coli and a glutaminase-free asparaginase from Vibri succinogenes on specific cell-mediated cytotoxicity. Int J Cancer 59—65 PubMed Google Scholar Furukawa S, Saito H, Fukatsu K, Hashiguchi Y, Inaba T, Lin M, Inoue T, Han I, Matsuda T, Muto T Glutamine-enhanced bacterial killing by neutrophils from postoperative patients. Nutrition — PubMed Google Scholar Garber AJ Glutamine metabolism in skeletal muscle. Academic Press, New York, pp — Google Scholar Griffiths M, Keast D The effect of glutamine on murine splenic leukocyte responses to T and B cell mitogens. Immunol Cell Biol — PubMed Google Scholar Griffiths RD, Jones C, Palmer TEA Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Am J Physiol E— PubMed Google Scholar Hammerqvist F, Wernerman J, von der Decken A, Vinnars E Alanyl-glutamine counteracts the depletion of free glutamine and post-operative decline in protein synthesis in muscle. Ann Surg — PubMed Google Scholar Haussinger D Glutamine metabolism in the liver: overview and current concepts. Metabolism 38 [Suppl 1]: 14—17 PubMed Google Scholar Herberer M, Babst R, Juretic A, Gross T, Horig H, Harder F, Spagnoli G Role of glutamine in the immune response in critical illness. Nutrition SS72 PubMed Google Scholar Hirsch EM L-glutaminase: suppression of lymphocyte blastogenic responses in vitro. Science — Google Scholar Houdijk APJ, Rijnsburger ER, Jansen J, Wesdorp RIC, Weis JK, McCamish MA, Teerlink T, Meuwissen SGM, Haarman HJTM, Thijs LG, van Leeuwen RAM Randomised trial of glutamine-enriched parenteral nutrition on infectious morbidity in patients with multiple trauma. Lancet — PubMed Google Scholar Inoue Y, Grant JP, Snyder PJ Effect of glutamine-supplemented intravenous nutrition on survival after Escherichia coli -induced peritonitis. JPEN 41—46 Google Scholar Jensen GL, Miller RH, Talabiska DG, Fish J, Gianferante L A double blind, prospective, randomized study of glutamine-enriched compared with standard peptide-based feeding in critically ill patients. Am J Clin Nutr — PubMed Google Scholar Kafkewitz D, Bendich A Enzyme-induced asparagine and glutamine depletion and immune system dysfunction. Am J Clin Nutr — PubMed Google Scholar Keast D, Newsholme EA Effect of mitogens on the maximum activities of hexokinase, lactate dehydrogenase, citrate synthase and glutaminase in rat mesenteric lymph node lymphocytes and splenocytes during the early period of culture. Int J Biochem — PubMed Google Scholar Keast D, Arstein DL, Harper W, Fry RW, Morton AR Depression of plasma glutamine concentration after exercise stress and its possible influence on the immune system. Med J Aust 15—18 PubMed Google Scholar Kew S, Wells SM, Yaqoob P, Wallace FA, Miles EA, Calder PC Dietary glutamine enhances murine T-lymphocyte responsiveness. J Nutr — PubMed Google Scholar Kweon MN, Moriguchi S, Mukai K, Kishino Y Effect of alanylglutamine-enriched infusion on tumour growth and cellular immune function in rats. Amino Acids 1: 7—16 Google Scholar Lacey JM, Wilmore DW Is glutamine a conditionally essential amino acid? Nutr Rev — PubMed Google Scholar Lowe DK, Benfell K, Smith RJ, Jacobs DO, Murawski B, Ziegler TR, Wilmore DW Safety of glutamine-enriched parenteral nutrient solutions in humans. Am J Clin Nutr — PubMed Google Scholar Lund J, Stjernstrom H, Bergholm U, Jorfeldt L, Vinnars E, Wiklund L The exchange of blood-borne amino acids in the leg during abdominal surgical trauma: effects of glucose infusion. Clin Sci — PubMed Google Scholar Lund P Metabolism of glutamine, glutamate and aspartate. Applied Sciences, London, pp — Google Scholar Lund P, Williamson DH Inter-tissue nitrogen fluxes. Br Med Bull — PubMed Google Scholar Max SR, Hill J, Mearow K, Konagaya H, Konagaya Y, Thomas JW, Banner C, Vitkavic L Dexamethasone regulates glutamine synthetase expression in rat skeletal muscles. Am J Physiol E—E PubMed Google Scholar Meister A Metabolism of glutamine. Physiol Rev — PubMed Google Scholar Milewski PJ, Threlfall CJ, Heath DF, Holbrook JB, Wilford K, Irving MH Intracellular free amino acids in undernourished patients with and without sepsis. Clin Sci 83—91 PubMed Google Scholar Morlion BJ, Stehle P, Wachter P, Siedhoff HP, Koller M, Konig W, Furst P, Puchstein C Total parenteral nutrition with glutamine dipeptide after major abdominal surgery — a randomized, double-blind, controlled study. Ann Surg — PubMed Google Scholar Muhlbacher F, Kapadia CR, Colpoys MF, Smith RJ, Wilmore DW Effects of glucocorticoids on glutamine metabolism in skeletal muscle. Am J Physiol EE83 PubMed Google Scholar Murphy C, Newsholme P Macrophage-mediated lysis of a β -cell line, tumour necrosis factor-a release from bacillus Calmette-Guerin BCG -activated murine macrophages and interleukin-8 release from human monocytes are dependent on extracellular glutamine concentrastion and glutamine metabolism. Clin Sci 89—97 PubMed Google Scholar Naka S, Saito H, Hashiguchi Y, Lin MT, Furukawa S, Inoba T, Fukushima R, Wada N, Muto T Alanyl-glutamine-supplemented total parenteral nutrition improves survival and protein metabolism in rat protracted bacterial peritonitis model. JPEN — Google Scholar Neu J, Roig JC, Meetze WH, Veerman M, Cater C, Millsaps M, Bowling D, Dallas MJ, Sleasman J, Knight T, Anestad N Enteral glutamine supplementation for very low birthweight infants decreases morbidity. J Pediatr — PubMed Google Scholar Newsholme EA, Newsholme P, Curi R, Crabtree B, Ardawi MSM Glutamine metabolism in different tissues: its physiological and pathological importance. Urban and Schwarzenberg, Baltimore, pp 71—98 Google Scholar Newsholme P, Newsholme EA Rates of utilisation of glucose, glutamine and oleate and formation of end products by mouse peritoneal macrophages in culture. Biochem J — PubMed Google Scholar Newsholme P, Curi R, Cordon S, Newsholme EA Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J — PubMed Google Scholar Newsholme P, Gordon S, Newsholme EA Rates of utilisation and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem J — PubMed Google Scholar Ogle CK, Ogle JD, Mao JX, Simon J, Noel JG, Li BG, Alexander JW Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. JPEN — Google Scholar O'Riordain M, Fearon KC, Ross JA, Rogers P, Falconer JS, Bartolo DCC, Garden OJ, Carter DC Glutamine supplemented parenteral nutrition enhances Tlymphocyte response in surgical patients undergoing colorectal resection. Ann Surg — PubMed Google Scholar O'Rourke AM, Rider LC Glucose, glutamine and ketone body utilisation by resting and concanavalin a activated rat splenic lymphocytes. Biochem Biophys Acta — PubMed Google Scholar Parry-Billings M, Leighton B, Dimitriadis GD, de Vasconcelos PRL, Newsholme EA Skeletal muscle glutamine metabolism during sepsis. Int J Biochem — PubMed Google Scholar Parry-Billings M, Evans J, Calder PC, Newsholme EA a Does glutamine contribute to immunosuppression after major burns? Lancet — PubMed Google Scholar Parry-Billings M, Leighton B, Dimitriadis GD, Bond J, Newsholme EA b Effects of physiological and pathological levels of glucocorticoids on skeletal muscle glutamine metabolism in the rat. Biochem Pharmacol — PubMed Google Scholar Parry-Billings M, Leighton B, Dimitriadis G, Curi R, Bond J, Bevan S, Colquhoun A, Newsholme EA The effect of tumour bearing on skleletal muscle glutamine metabolism. Int J Biochem — PubMed Google Scholar Parry-Billings M, Baigrie RJ, Lamont PM, Morris PJ, Newsholme EA a Effects of major and minor surgery on plasma glutamine and cytokine levels. Arch Surg — PubMed Google Scholar Parry-Billings M, Budgett R, Koutedakis Y, Blomstrand E, Williams C, Calder PC, Pilling S, Baigrie R, Newsholme EA b Plasma amino acid concentrations in the overtraining syndrome: possible effects on the immune system. Med Sci Sports Exerc — PubMed Google Scholar Peltonen E, Pulkki K, Kirvela O Stimulatory effect of glutamine on human monocyte activation as measured by interleukin-6 and soluble interleukin-6 receptor release. Clin Nutr — Google Scholar Powell H, Castell LM, Parry-Billings M, Desborough JP, Hall GM, Newsholme EA Growth hormone suppression and glutamine flux associated with cardiac surgery. Clin Physiol — PubMed Google Scholar Rohde T, Maclean DA, Hartkopp A, Pedersen BK a The immune system and serum glutamine during a triathalon. Eur J Appl Physiol — Google Scholar Rohde T, Maclean DA, Pedersen BK b Glutamine, lymphocyte proliferation and cytokine production. Scand J Immunol — PubMed Google Scholar Roth E, Funovics J, Muhlbacher F, Schemper M, Mauritz W, Sporn P, Fritsch A Metabolic disorders in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr 1: 25—41 Google Scholar Scheltinga MR, Young LS, Benfell K, Bye RL, Ziegler TR, Santos AA, Antin JH, Schloerb PR, Wilmore DW Glutamine-enriched intravenous feedings attenuate extracellular fluid expansion after standard stress. Ann Surg — PubMed Google Scholar Schroder J, Lahlke V, Fandrich F, Gebhardt H, Erichsen H, Zabel P, Schroeder P Glutamine dipeptides-supplemented parenteral nutrition reverses gut muscosal structure and interleukin-6 release of rat intestinal mononuclear cells after hemorrhagic shock. Shock 26—31 PubMed Google Scholar Shewchuk LD, Baracos VE, Field CJ Dietary L-glutamine supplementation reduces growth of the Morris Hepatoma in exercise-trained and sendentary rats. J Nutr — PubMed Google Scholar Simberkoff MS, Thomas L Reversal by L-glutamine of the inhibition of lymphocyte mitosis caused by E. Proc Soc Exp Biol — Google Scholar Smith KA Interleukin inception, impact and implications. Science — PubMed Google Scholar Souba WW, Smith RJ, Wilmore DW Glutamine metabolism in the intestinal tract. JPEN 9: — Google Scholar Souba WW, Klimberg VS, Hautamaki RD, Mendenhall WH, Bova FC, Howard RJ, Bland KI, Copeland III EM Oral glutamine reduces bacterial translocation following abdominal radiation. J Surg Res 1—5 PubMed Google Scholar Spittler A, Winkler S, Gotzinger P, Oehler R, Willheim M, Tempfer C, Weigel G, Fugger R, Boltz-Nitulescu G, Roth E Influence of glutamine on the phenotype and function of human monocytes. Blood — PubMed Google Scholar Spittler A, Holzer S, Oehler R, Boltz-Nitulescu G, Roth E A glutamine deficiency impairs the function of cultured human monocytes. Clin Nutr 97—99 Google Scholar Stehle P, Zander J, Mertes N, Albers S, Puchstein C, Lavin P, Furst P Effect of parenteral glutamine dipeptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet i: — Google Scholar Stinnett JD, Alexander JW, Watanabe C, Elwyn DH, Furst P, Kantrowitz LR, Gump FE, Kinney JM Plasma and skeletal muscle amino acids following severe burn injury in patients and experimental animals. Ann Surg 75—89 PubMed Google Scholar Suzuki I, Matsumoto Y, Adjei AA, Osato L, Shinjo S, Yamamoto S Effect of a glutamine-supplemented diet in response to methicellin-resistant Staphylococcus aureus infection in mice. J Nutr Sci Vitaminol — PubMed Google Scholar Szondy Z, Newsholme EA The effect of glutamine concentration on the activity of carbamoyl-phosphate synthase II and on the incorporation of [ 3 H]thymidine into DNA in rat mesenteric lymphocytes stimulated by phytohaemagglutinin. Biochem J — PubMed Google Scholar Tizianello A, Deferrari G, Garibotto G, Robabaudo C, Asquarone N, Ghiggeri GN Renal ammoniagenesis in an early stage of metabolic acidosis in man. J Clin Invest — PubMed Google Scholar van der Hulst RRW, van Kreel BK, von Meyenfeldt MF, Brummer R-JM, Arends J-W, Deutz NEP, Soeters PB Glutamine and the preservation of gut integrity. Lancet — PubMed Google Scholar Wallace C, Keast D Glutamine and macrophage function. Metabolism — PubMed Google Scholar Wells SM, Kew S, Yaqoob P, Wallace FA, Calder PC Dietary glutamine enhances cytokine production by murine macrophages. Nutrition in press Windmeuller HG, Spaeth AE Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem — PubMed Google Scholar Yoshida S, Hikida S, Tanaka Y, Yanase A, Mizote H, Kaegawa T Effect of glutamine supplementation on lymphocyte function in septic rats. JPEN 30S Google Scholar Yaqoob P, Calder PC Glutamine requirement of proliferating T lymphocytes. Nutrition — PubMed Google Scholar Yaqoob P, Calder PC Cytokine production by human peripheral blood mononuclear cells: differential sensitivity to glutamine availability. Cytokine — PubMed Google Scholar Yoo SS, Field CJ, McBurney MI Glutamine supplementation maintains intramuscular glutamine concentrations and normalizes lymphocyte function in infected early weaned pigs. J Nutr — PubMed Google Scholar Ziegler TR, Bye RL, Persinger RL, Young LS, Antin JH, Wilmore DW Effects of glutamine supplementation on circulating lymphocytes after bone marrow transplantation: A pilot study. Am J Med Sci 4—10 PubMed Google Scholar Ziegler TR, Young LS, Benfell K, Scheltinga M, Hortog K, Bye R, Morrow FD, Jacobs DO, Smith RJ, Antin JH, Wilmore DW Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition following bone marrow transplantation: a double-blinded, randomized, controlled trial. Ann Intern Med — PubMed Google Scholar Download references. Some recent studies have shown that there were same regulatory molecules enabling the crosstalk between glutamine metabolism and the expression of immune checkpoint proteins. As previously highlighted, the proto-oncogene Myc has been shown to be critical for glutamine metabolism. MYC specifically activates the expression of glutamine transporter and glutaminase in tumors, thereby regulating the reprogramming of glutamine metabolism in tumors [ 30 , 72 , 73 ]. At the same time, MYC also regulates the expression of PD-L1 and CD47 in the tumor cells. MYC expressed by the tumor cells not only regulates the tumor immune microenvironment by acting on innate and acquired immune cells and the secretion of cytokines, but also by direct action on the promoters of the genes encoding CD47 and PD-L1, which in turn regulates their mRNA and protein expression, eventually causing immunosuppression and tumor growth [ 74 ]. The Ras oncogene promotes the reprogramming of glutamine metabolism in tumor cells by up-regulating the expression of glutaminase [ 75 ]. Mutation of the K-Ras gene activates the downstream signaling pathway involved in stabilizing the PD-L1 mRNA, thereby promoting PD-L1 protein synthesis by tumor cells and inhibiting the anti-tumor immune response [ 76 ]. In addition to Myc and Ras , HIF and p53 have also been shown to be involved in the regulation of glutamine metabolism in tumors, as well as in the expression of immune checkpoints by tumor cells [ 77 , 78 ]. Although many researches have confirmed that some of the same regulators are involved in mediating both the regulation of glutamine metabolism and immune checkpoints, these studies were independent and did not link glutamine metabolism with immune checkpoints expression. Therefore, whether these factors regulate the expression of immune checkpoints while regulating glutamine metabolism in tumors and thus affect the anti-tumor immune response needs to be further explored. Energy metabolism is an important basis for maintaining the activity and function of immune cells. In the process of immune cell activation, a large amount of energy and metabolic intermediates are required to meet the needs of macromolecule biosynthesis, so as to achieve cell proliferation, differentiation and effector functions. Glutamine is an important energy substrate for immune cells, and an important nitrogen and carbon donor for various biosynthetic precursors, and also plays a critical role in the activation and function of immune cells. Therefore, it is crucial to understand how changes in glutamine metabolism in immune cells affects their anti-tumor immune responses. T cells are key players in the anti-tumor immune response. Usually in the resting state, the metabolic rate of the naive T cells is low; its demand for glutamine is low; and low levels of glutamine metabolism can maintain its survival [ 81 ]. However, in the activated state, the Teff cells need to proliferate rapidly, thus increasing the intake of glutamine, which provides them with sufficient raw material for macromolecule synthesis, while promoting the secretion of cytokines [ 82 ]. The decomposition of glutamine affects the differentiation of T cells. Additionally, the loss of GlS1 leads to α-kG deficiency, and impairs the differentiation of Th17 cells [ 84 ]. During glutamine deprivation, Teff cells show decreased c-MYC protein expression, growth restriction, and impaired immune function [ 85 ]. In addition, glutamine deprivation promotes the differentiation of Treg cells through AMPK-mTORC1 signaling pathway, thereby reducing the immune function of Teff cells [ 86 , 87 ]. In summary, the reprogramming of glutamine metabolism in T cells regulates the differentiation and function of T cells from various aspects, thereby regulating the immune response of the body Fig. Macrophages are innate immune cells. Under the stimulation of lipopolysaccharide LPS and IFN-γ or IL-4, naive macrophages differentiate into M1 or M2 macrophages. M1 macrophages participate in the positive immune responses and play the role in immune surveillance by secreting inflammatory cytokines and chemokines, and are involved in professional antigen presentation. M2 macrophages possess a weak antigen-presenting ability, and secrete anti-inflammatory cytokines such as IL or TGF-β, and down-regulate the immune response [ 88 , 89 , 90 ]. Tumor-associated macrophages TAMs have been shown to be functionally plastic as a special type of macrophage, which are often described as M2-like population, but there is also evidence for the existence of M1-like population [ 91 , 92 , 93 ]. In fact, in the early phase of tumor establishment, TAMs display an inflammatory phenotype, but an immunosuppressive phenotype is present at the later stages of tumor progression [ 94 ]. Glutamine metabolism plays an important role in the activation of macrophages, and there are inherent differences in the dependence of different macrophage subsets on glutamine. For example, early in vivo animal experiments showed that glutamine was essential for the production of cytokines such as IL-1, IL-6, TNFα , antigen presentation, and phagocytic functions in murine macrophages [ 50 ]. Glutaminolysis affects the polarization of M1 macrophages. The uptake and metabolism of glutamine is elevated in LPS-activated M1 macrophages, and the replenishment of α-KG by glutamine metabolism further promotes the accumulation of succinate, improving the stability of HIF-1α, which in turn drives the production of pro-inflammatory cytokines such as IL-1 [ 95 , 96 ]. M2 macrophages consume more glutamine than M1 and naive macrophages, and usually glutamine accumulates in M2 macrophages and promotes its polarization. Part of the reason for this differential effect is that the metabolite of glutamine, α-kG, alters gene expression programs that support an anti-inflammatory M2-like state. Additionally, the expression of glutamine synthase GS is low in M1 macrophages, but high in M2 macrophages [ 58 , 97 , 98 ]. Recent studies have shown that in ILinduced M2 macrophages, glutamine is used to support active TCA cycle, and HBP. The HBP pathway produces UDP-GlcNAc, which acts as a substrate for N-glycosylation of M2-marked proteins, such as the N-glycosylation receptor CD, as well as KLF4, CCL22, and IRF4, thereby promoting the polarization of M2 macrophages [ 96 ]. Supporting these observations, TAMs from Lewis lung cancer M2 phenotype was reported to express higher levels of the glutamine metabolizing enzymes, transaminase and glutamine synthetase. However, whether and how glutamine metabolism regulates the tumor-promoting function of TAMs remains to be further demonstrated [ 99 ]. Taken together, glutamine metabolism is involved in the polarization of M1 and M2 macrophages. Since M2 macrophages consume more glutamine than M1 macrophages, in the TME, it is unclear whether inhibiting the anti-tumor immune response from M2 macrophages polarization would be greater than the effect of enhancing the anti-tumor immune response from the M1 macrophages. Or is there a homeostasis between the M1 and M2 states. Furthermore, it is not known whether glutamine metabolism affects the differentiation of naive macrophages. All the above factors would determine whether the glutamine metabolism in macrophages promotes or inhibits the anti-tumor immune response Fig. Impact of reprogramming of glutamine metabolism in immune cells on immune response. M2 macrophages consume more glutamine, and α-KG, a metabolite of glutamine metabolism, which promote the polarization of M2 macrophages. Glutamine metabolism in M2 macrophages is essential for supporting an active TCA cycle and UDP-GlcNAc synthesis. B cells modulate the function of myeloid cells to support tumor progression, by producing antibodies and immune complexes [ , ]. Glutamine is essential for the survival of B cells in hypoxic environments [ ], and also promotes the differentiation of human B cells into plasma cells and lymphocytes [ 57 ]. In addition, antibody production by B cells depends on the breakdown of glutamine. When the expression of ASCT2 and GLS are inhibited, the production of IgG and IgM antibodies is reduced [ ]. Neutrophils consume glutamine at the highest rates relative to other leukocytes, such as macrophages and lymphocytes [ , ]. Glutamine enhances superoxide production in neutrophils by generating ATP and regulates the expression of components of the NADPH oxidase complex [ ]. Furthermore, glutamine plays an important role in preventing adrenaline induced changes in NADPH oxidase and superoxide production in neutrophils [ ]. NADPH oxidase is essential for neutrophil function, as neutrophils use extracellular traps NETs to perform their functions, and the action of NETs requires the activation of NADPH oxidase [ ]. Therefore, glutamine metabolism is crucial for the function of neutrophils, but its pro-tumor or anti-tumor effects need to be further explored. Natural killer NK cells play an integral role in activating anti-tumor T cell responses and killing tumor cells by producing IFN-γ and cytotoxic molecules such as granzyme. Glutamine uptake mediated by the glutamine transporter SLC7A5, regulates the activation of c-MYC-dependent NK cells. When glutamine is deprived, NK cells exhibit reduced expression of c-MYC protein, growth restriction, and impaired immune function, while inhibition of glutamine breakdown has no effect on NK cells [ 85 ]. In addition to the immune cells discussed above, other immune cells have also been shown to play an important role in the anti-tumor immune response. Glutamine in the TME not just meets the metabolic needs of the rapidly proliferating tumor cells, but also does the same for the different types of immune cells. As mentioned above, the differential impact of glutamine metabolism on different types of cells in the TME would eventually determine the outcome of targeting glutamine metabolism, and its effect on tumor suppression and anti-tumor immune response. The current drugs targeting glutamine metabolism are mainly classified into three categories, namely, glutamine antimetabolites, glutaminase inhibitors and glutamine uptake inhibitors. Several recent studies have demonstrated that the above three classes of glutamine metabolism inhibitors positively impact the function of different immune cells in the TME, while inhibiting tumor cell proliferation. Although it promotes a strong anti-tumor effect, systemic toxicity limits its clinical application [ , , ]. To address this problem, researchers developed JHU, a prodrug form of L-DON, which is selectively activated to L-DON after entering the TME, thus reducing its systemic toxicity and improving its anti-tumor immune response [ , ]. In syngeneic mouse models treated with JHU, the metabolic activity of the tumor was extensively suppressed, while hypoxia was mitigated and the levels of glutamine and glucose in the TME were increased. Therefore, it is proposed that JHU may not have negative effects on immune cells, but may enhance the function of immune cells. Mechanistically, L-DON and JHU suppressed tumor glutamine metabolism by inhibiting all the glutamine-utilizing enzymes, and simultaneously suppressed tumor glycolysis by activating AMP Kinase AMPK and inhibiting the expression of c-MYC [ , ]. AMPK and c-MYC are recognized as key regulators of glycolytic flux [ , , ]. Furthermore, OXPHOS in tumor cells was also suppressed due to the absence of alternative fuels as carbon sources for the TCA cycle [ ]. Overall, due to the lack of plasticity in the interdependence of glycolysis, OXPHOS and glutamine metabolism in tumor cells, extensive inhibition of glutamine metabolism in tumor cells inhibits their glycolysis and OXPHOS, thereby comprehensively disintegrating the energy metabolism in tumor cells [ ]. Generally, myeloid-derived suppressor cells MDSCs and TAMs in the TME inhibit the anti-tumor immune response. In tumor-bearing mouse models treated with JHU, tumor growth was found to be suppressed and the generation and recruitment of MDSCs was also markedly inhibited. Mechanistically, targeting tumor glutamine metabolism in the tumors promoted a decrease in CSF3secretion, and promoted the differentiation of MDSCs and TAMs into pro-inflammatory TAMs. Additionally, blocking glutamine metabolism also inhibited the expression of IDO in the tumor and myeloid derived cells, resulting in a significant reduction in the levels of kynurenine, further enhancing the anti-tumor immune response [ ]. Interestingly, L-DON also enhanced the anti-tumor immune response by affecting the mechanical properties of the tumor extracellular matrix ECM , which is responsible for the formation of the immunosuppressive TME. In conclusion, glutamine antimetabolites effectively inhibit tumor growth while improving the anti-tumor immune response through multiple mechanisms, revealing the close interaction between glutamine metabolism and immune response in the TME Fig. Effects of glutamine metabolism inhibitors on immune response. GLS is highly expressed in diverse malignancies and is essential for their survival, and tumor-targeted drugs targeting GLS have been extensively studied. BPTES and , are the two major classes of GLS inhibitors that have been shown to have anti-tumor activity [ 42 , ]. CB, a BPTES-based allosteric GLS inhibitor, with a better oral bioavailability and stronger inhibitory activity, is being tested in clinical trials [ ]. However, BPTES treatment also promoted the upregulation of PD-L1 expression, which inhibited the anti-tumor function of immune cells upon PD-L1 binding to the PD-1 receptor on the surface of immune cells. Therefore, there is the possibility of immune escape after treating tumor cells with BPTES [ 71 ]. CB has varied effects on the function of different types of T cells, thereby affecting the immune response. However, treatment of Th17 cells with CB inhibited their differentiation, function, and cytokine production, and eventually suppressed their expansion. The reason for these opposing outcomes may be due to differences in the epigenetic response of each T cell subset to α-KG depletion after GLS blockade [ 65 , 83 ]. However, it did not lead to a long-term effect. In addition, transient exposure to CB in vitro improved the function of chimeric antigen receptor CAR T cells, in a mouse model receiving CAR-T cell immunotherapy for a limited time [ 83 ]. Therefore, the glutaminase inhibitor CB enhances the function of Teff cells while inhibiting the function of Treg cells, thereby enhancing the anti-tumor immune response Fig. The glutamine transporter SLC1A5, is frequently up-regulated in the tumor cells, and its overexpression is associated with a poor prognosis in cancer patients [ 30 , ]. V, an inhibitor targeting SLC1A5, significantly inhibits tumor cells proliferation in vitro, and suppresses tumor growth in mouse models, and also modulates the anti-tumor immune response [ ]. In a spontaneous mouse model of TNBC, researchers found that V selectively blocked glutamine uptake in TNBC cells to inhibit tumor growth, but did not inhibit the T cells. However, such compensatory upregulation of glutamine transport was not found in Vtreated TNBC cells [ 17 ]. Meanwhile, in human breast cancer cell lines, researchers found that V enhanced anti-tumor response by promoting ROS production, which induced the autophagic degradation of B7 homology 3 B7H3. B7H3 is considered to act as an immune checkpoint ligand that contributes to immune escape. The combination of V and anti-PD-1 antibody showed a greater anti-tumor effect than either of the single treatments [ , ]. Consistent with BPTES, tumor cells treated with V up-regulated the expression of PD-L1, Thus, there is the possibility of immune escape after treating tumor cells with V, and the combined targeting of glutamine metabolism and PD-L1 showed a greater anti-tumor efficacy in mouse tumor models [ 71 ]. The critical role of glutamine in energy generation and macromolecule synthesis underlies its importance in tumor progression and immune response. Therefore, further studies exploring the role of glutamine metabolism in the tumors and immune cells would help us to develop therapeutic strategies for targeting glutamine metabolism in the TME for cancer therapy. In fact, both tumor cells and immune cells greatly depend on the availability of glutamine to survive, proliferate, and function. Reprogramming of glutamine metabolism in the tumor cells to their biosynthetic and energy requirements to support their rapid proliferation and survival in a hypoxic TME. And reprogramming of glutamine metabolism in the immune cells maintains their survival while modulating their phenotypes and function, contributing to their pro-tumorigenic or anti-tumorigenic functions. For example, there may be a competition for glutamine between immune and tumor cells, and cell-programmed glutamine partitioning may happen between these cells in the TME. Glutamine metabolism affects immune response by regulating the differentiation and activity of Teff cells, Treg cells, and macrophages, and the expression of immune checkpoint proteins in tumor cells. Also, different types of glutamine metabolism inhibitors have been reported to have varying effects on different immune cells, while inhibiting the proliferation of tumor cells. Together, the above factors determine how glutamine metabolism in the TME affects the immune response, eventually causing tumor progression or suppression. Combination therapy with immunotherapeutic agents and drugs targeting tumor metabolism are a major focus of current cancer research. Studies have reported a synergistic effect of targeting glutamine metabolism and anti-tumor immunity. However, the current clinical trials related to the above combination therapy have not achieved satisfactory results [ ]. The reasons for this may include the inherent heterogeneity in glutamine metabolism in the tumor and immune cells, and the intricate effects of glutamine metabolism on immune responses. As discussed in our paper, glutamine uptake inhibitors not only activate the function of Teff cells in the TME, but also up-regulate the expression of PD-L1 in tumor cells, which may inhibit the function of Teff cells. This indicates the complexity of the effects of glutamine metabolism on immune responses. Although the current studies have confirmed that various types of glutamine metabolism inhibitors not just inhibit tumor proliferation effectively, but also have a positive impact on the anti-tumor function of immune cells. However, one needs to evaluate whether they also modulate the expression of immune checkpoint proteins in tumor cells and contribute to immune escape. Therefore, a deeper investigation of glutamine metabolism in tumor cells and immune cells, and the crosstalk between these cells would help us understand the mechanisms associated with immune evasion, and the glutamine requirements by immune cells, which would be crucial to fully realize the effect of combination therapy. Data sharing is not applicable to this article as no new data were created or analyzed in this study. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Article CAS PubMed Google Scholar. Faubert B, Solmonson A. Metabolic reprogramming and cancer progression. Article PubMed PubMed Central Google Scholar. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Article CAS PubMed PubMed Central Google Scholar. McCarty MF, Whitaker J. Manipulating tumor acidification as a cancer treatment strategy. Altern Med Rev. PubMed Google Scholar. Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo JH, Kim JK, Heo Y, Lee HS, Lee MY, et al. A variant of SLC1A5 Is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. Huang W, Choi W, Chen Y, Zhang Q, Deng H, He W, Shi Y. A proposed role for glutamine in cancer cell growth through acid resistance. Cell Res. Yang T, Yan X, Cao Y, Bao T, Li G, Gu S, Xiong K, Xiao T. Meta-analysis of glutamine on immune function and post-operative complications of patients with colorectal cancer. Front Nutr. Article PubMed PubMed Central CAS Google Scholar. Kaur BP, Secord E. Innate Immunity. Pediatr Clin North Am. Article PubMed Google Scholar. Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. Terry S, Engelsen AST, Buart S, Elsayed WS, Venkatesh GH, Chouaib S. Hypoxia-driven intratumor heterogeneity and immune evasion. Cancer Lett. Huang B, Song BL. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. Chen B, Gao A, Tu B, Wang Y, Yu X, Wang Y, Xiu Y, Wang B, Wan Y, Huang Y. Metabolic modulation via mTOR pathway and anti-angiogenesis remodels tumor microenvironment using PD-L1-targeting codelivery. Karayama M, Masuda J, Mori K, Yasui H, Hozumi H, Suzuki Y, Furuhashi K, Fujisawa T, Enomoto N, Nakamura Y, et al. Comprehensive assessment of multiple tryptophan metabolites as potential biomarkers for immune checkpoint inhibitors in patients with non-small cell lung cancer. Clin Transl Oncol. Yan Y, Chang L, Tian H, Wang L, Zhang Y, Yang T, Li G, Hu W, Shah K, Chen G, Guo Y. J Immunother Cancer. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Edwards DN, Ngwa VM, Raybuck AL, Wang S, Hwang Y, Kim LC, Cho SH, Paik Y, Wang Q, Zhang S, et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J Clin Invest. Wang JX, Choi SYC, Niu X, Kang N, Xue H, Killam J, Wang Y. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci. Article CAS PubMed Central Google Scholar. Ma G, Li C, Zhang Z, Liang Y, Liang Z, Chen Y, Wang L, Li D, Zeng M, Shan W, Niu H. Front Oncol. Carrascosa JM, Martínez P, Núñez de Castro I. Nitrogen movement between host and tumor in mice inoculated with Ehrlich ascitic tumor cells. Cancer Res. CAS PubMed Google Scholar. Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. Daye D, Wellen KE. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol. Welbourne TC. Ammonia production and glutamine incorporation into glutathione in the functioning rat kidney. Can J Biochem. DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal. Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y, et al. Phosphate-activated glutaminase GLS2 , a pinducible regulator of glutamine metabolism and reactive oxygen species. Jackson JG, Lozano G. The mutant p53 mouse as a pre-clinical model. Tran TQ, Lowman XH, Reid MA, Mendez-Dorantes C, Pan M, Yang Y, Kong M. Tumor-associated mutant p53 promotes cancer cell survival upon glutamine deprivation through p21 induction. Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Dilshara MG, Jeong JW, Prasad Tharanga Jayasooriya RG, Neelaka Molagoda IM, Lee S, Park SR, Choi YH, Kim GY. Glutamine deprivation sensitizes human breast cancer MDA-MB cells to TRIAL-mediated apoptosis. Biochem Biophys Res Commun. Gaglio D, Soldati C, Vanoni M, Alberghina L, Chiaradonna F. Glutamine deprivation induces abortive s-phase rescued by deoxyribonucleotides in k-ras transformed fibroblasts. PLoS ONE. Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C, Alberghina L, Stephanopoulos G, Chiaradonna F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol. Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. Faubert B, Vincent EE, Griss T, Samborska B, Izreig S, Svensson RU, Mamer OA, Avizonis D, Shackelford DB, Shaw RJ, Jones RG. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1α. Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, Cerione RA. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Ghesquière B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Article PubMed CAS Google Scholar. |
Und was jenes zu sagen hier?
Ich denke, dass Sie den Fehler zulassen. Ich kann die Position verteidigen.
Welcher neugierig topic